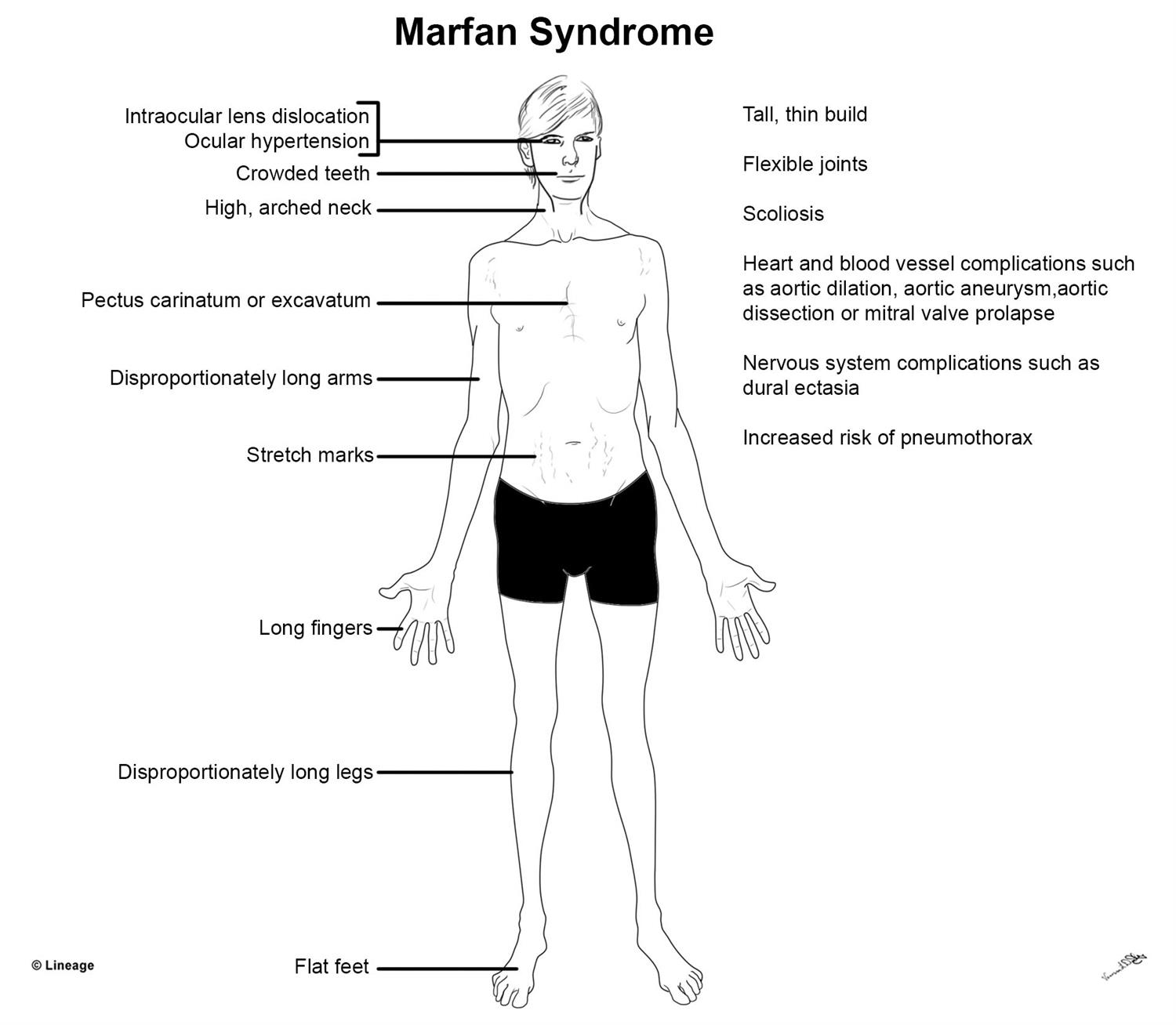
We perform comprehensive bioinformatics testing on DNA RNA and proteins at our state-of-the-art 66000 sq. Our partnership with Ambry Genetics allows the ability to order Caris Molecular Intelligence and Ambrys CancerNext- Expanded test from one service provider and receive a consolidated report with both somatic and germline results.
 Caris Molecular Intelligence Tumor Profiling Enabling Precision Medicine
Caris Molecular Intelligence Tumor Profiling Enabling Precision Medicine
The overarching concept of Caris Molecular Intelligence has enabled Caris to advance precision medicine through comprehensive tumor profiling of DNA RNA and protein biomarkers revealing the highest quality molecular blueprints for selection of the most appropriate cancer therapeutic regimens.

Caris molecular testing. Caris Molecular Intelligence tumor profiling includes microsatellite instability MSI testing via next-generation sequencing NGS. Our Comprehensive Tumor Profiling approach to assess DNA RNA and Proteins reveals a molecular blueprint to guide more precise and individualized treatment decisions. This information may be a powerful tool to aid oncologists in personalizing cancer therapies for their patients.
Identify clinical trial opportunities based on biomarker expression with. Caris Molecular Intelligence enables the delivery of precision medicine by providing oncologists with clinically actionable and individualized cancer treatment information to help personalize cancer care. Better information can lead to better treatment decisions.
The Caris Molecular Intelligence test is very innovative in that it analyzes a patients tissue for specific genetic alterations. 8 Feb 2020 1453. Patients with a family history of breast cancer or other tumors bilateral breast cancers or early-onset breast cancers warrant genetic testing to determine whether a hereditary cancer syndrome is present.
CMI uses a number of tumour profiling techniques to analyse protein RNA and DNA in the tumour. In fewer than 14 days Caris Life Sciences assesses and packages clinically relevant information to help healthcare providers inform the creation of medical therapies that are more effective because they are tailored to specific cancer. Caris Molecular Intelligence CMI is a test that is used to help guide management of advanced cancer patients fit for further treatment based on clinical ECOG-status estimated life-expectancy and quality of life.
Tests indicated on the requisition the Caris pathologist will prioritize and order the appropriate testing unless otherwise indicated by the ordering physician. The Caris Molecular Intelligence comprehensive tumor profiling approach to assess DNA RNA and proteins reveals a molecular blueprint to guide more precise and individualized treatment decisions from about 60 FDA-approved therapies. Caris Life Sciences is committed to delivering the promise of personalized medicine.
MSI is caused by failure of the DNA mismatch repair MMR system. Its possible that molecular testing is doing a lot of good pinpointing cancer therapies that are most likely or least likely to work. The latter picture is painted in a suit filed by two former employees of Caris Life Sciences Inc a company that.
Order services with pre-populated requisitions physician and practice information Download full and summary reports securely. It is intended as a tool to aid decision-making and help identify the best treatment plan for each patient. Its also possible that Medicare is paying for molecular tests that are marketed aggressively despite being based on flimsy evidence.
The first of its kind Caris Molecular Intelligence is a unique tool that combines comprehensive tumor testing with the latest clinical research on biomarkers to provide your doctor with timely information to evaluate potential treatment options. If If limited tissue communication is requested before moving forward with testing Caris Life Sciences will fax the ordering physician the proposed list of tests. At our 66000 square foot state-of-the-art laboratory located in Phoenix Arizona we provide high quality reliable molecular testing services for all stages of the drug development cycle and routine clinical use.
CMI was marketed as Target Now until 2013. Track real-time case status. With just a few clicks users can.
Laboratory in Phoenix Arizona. High levels of MSI correlate to an increased neoantigen burden which may make the tumor more sensitive to immunotherapy. The Caris Molecular Intelligence comprehensive tumor profiling approach to assess DNA RNA and Proteins reveals the highest quality molecular blueprint to guide more precise and individualized treatment decisions that is proven to extend overall survival.
A surgical or biopsy specimen is obtained from the pathology lab by Caris and an in-depth analysis is performed to evaluate the presence and activity level of hundreds of genes. Caris molecular testing. Mutations are identified and these mutations are used to guide treatment.
As the most experienced and comprehensive tumor profiling service we take pride in our industry leading. How does Caris Molecular Intelligence work. My husband has just started chemo for choriocarcinoma - usually in pregnant women - so it is very unusual in a man - following excision of malignant tumour on his left adrenal gland.
Caris Molecular Intelligence CMI Caris Life Sciences is a solid tumour biomarker analysis service. From pre-clinical research for compound development efforts to established commercially available therapies Caris provides robust genomic and proteomic testing. Caris has three clinical lab facilities in the United States all CLIA.
Caris Molecular Intelligence is an analysis of a cancerous tumor at the molecular level that helps to make the most detailed profile of the malignant tissue on the basis of which our oncologists can recommend to the patient drugs actually effective on his tumor and also to determine the list of drugs that will not be effective. Molecular testing for genetic and genomic variation has become an integral part of breast cancer management. Caris Molecular Intelligence performs comprehensive molecular testing on the DNA RNA and Proteins to identify the biomarkers driving a patients tumor.