Compared to tissue genotyping n5621 used in GI-SCREEN the Guardant360 liquid biopsy n1687 shortened screening duration by 67 percent median 11 vs. Guardant360 CDx is a lab test that detects genetic mutations found in circulating cell-free DNA cfDNA to help doctors identify patients with non-small cell.
 With Its Liquid Biopsy Approval Foundation Medicine Takes On Guardant Evaluate
With Its Liquid Biopsy Approval Foundation Medicine Takes On Guardant Evaluate
For example Guardant wrote in its filing Foundation has claimed in its marketing that clinical data show Guardant360 detects cancer-related genomic alterations in only 58 percent of patients while Foundations tissue test FoundationOne detects cancer-related genomic alterations in up to 98 percent of patients tested.

Guardant 360 vs foundation one. Investigators studied a variety of factors and their association with immunotherapy response including microsatellite instability MSI and PD-L1 expression but also tumor mutational burden more specifically whether Guardants 360 assay could recapitulate the same response prediction as tissue-based measurements of TMB. Guardant360 CDx made by Guardant Health was approved as a companion diagnostic for osimertinib Tagrisso a lung cancer therapy. Comprehensive support and educational resources for you and your patients.
The FoundationOne F1. In this study bTMB and tTMB were highly correlated in TMB high patients. Medicare-covered for all solid tumors regardless of tissue availability.
But none of these pan-cancer blood assays is approved by the FDA all are marketed as lab-developed tests. Genomic Profiling Using Guardant 360 Cell-Free DNA-Based Assay vs Tumor-Based Genotyping Assays in Advanced NSCLC. Silicon Valley-based Guardant Health was founded in 2012 by a team of serial entrepreneurs.
Foundation One starts with a tissue biopsy while Guardant360 starts with a blood sample. One final factor to consider is explored in a 2019 study led by Merck where bTMB analysis from both FMI and Guardant health are compared to tTMB as measured in WES 8. Redwood City Calif-based Guardant Health is taking Roche-owned Foundation Medicine to court over a patent dispute for their liquid biopsy technology further heating up the rivalry between the two cancer detection companies.
Seamlessly integrate Guardant360 CDx into your practice. Foundation Medicine test sequences clinical tumor samples to characterize the exons of 315 cancer-associated genes and introns from 28 genes involved in rearrangements. Nov 25 2020 By Brandon May.
Guardant Health is leading the way for liquid biopsy to help patients across the cancer care continuum In 2014 we introduced the Guardant360 test for advanced cancer patients. FoundationOne Liquid CDx made by Foundation Medicine was approved as a companion diagnostic for three lung cancer therapies and a. One company that is looking to offer both cancer blood testing and personalized cancer genomics reports is Guardant Health.
Our test overcame the challenges of. It was the first-in-kind liquid biopsy to comprehensively sequence a patients cancer to reveal actionable mutations. The CEO Helmy Eltoukhy co-founded Avantome in 2007 which was sold to Illumina one year later for 60 million.
One chunk may have cells with mutation A while another chunk has cells with mutation B. 2 Giving the likely limited benefit. Guardant Health and Foundation Medicine Face Off in Another Legal Battle.
Guardant Health test uses cell-free circulating DNA from blood to sequence 70 genes. Complete genomic results in 7 days. Claims that Guardants test infringes on Foundations patent.
A cutting-edge cancer testing company is suing one of its biggest rivals. In newly released documents Guardant claims Foundation is currently infringing on up to seven patents which are part of Guardants. 1 Due to limitations interpreting results most NET oncologists are not inclined to alter your plan of care based on these sorts of genetic profiling assays.
In other words despite all the hoopla in the news the data provided by either test is usually not actionable for NET patients at the present time. But Guardants and Foundations tests both cost 5800 and most insurance policies including. First FDA-approved comprehensive liquid biopsy.
Trial Results The Roche subsidiary Foundation Medicine launched its liquid biopsy FoundationOne Liquid in the US in September whereupon the test joined Guardant Healths Guardant360 among others in a fast-developing market.
 Precision Medicine Part I The Science Behind Molecular Testing Blue Matter Consulting
Precision Medicine Part I The Science Behind Molecular Testing Blue Matter Consulting
Http Simposiobiopsialiquida Com Wp Content Uploads 2018 01 2 Dr Emiliano Calvo Pdf
 With Its Liquid Biopsy Approval Foundation Medicine Takes On Guardant Evaluate
With Its Liquid Biopsy Approval Foundation Medicine Takes On Guardant Evaluate
 Aacr 2019 Foundation Medicine Sets The Scene For Liquid Biopsy Approval Evaluate
Aacr 2019 Foundation Medicine Sets The Scene For Liquid Biopsy Approval Evaluate
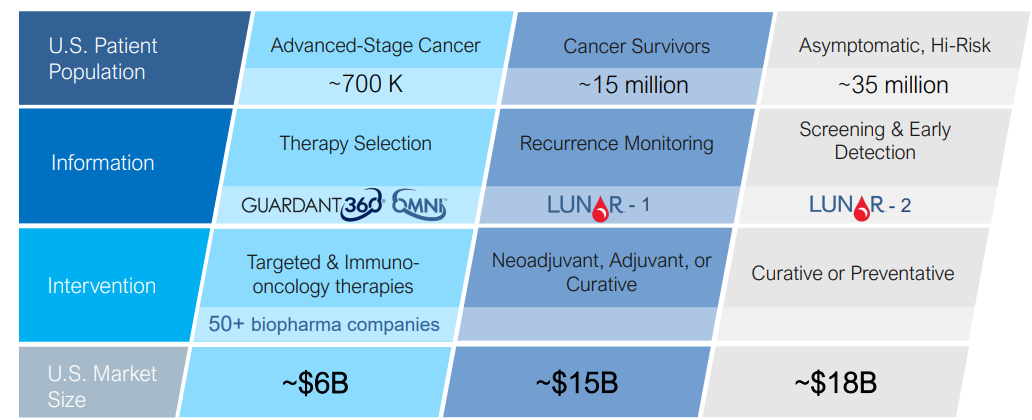 Guardant Health Bright Future For Liquid Biopsy Leader But Valuation Is Rich Nasdaq Gh Seeking Alpha
Guardant Health Bright Future For Liquid Biopsy Leader But Valuation Is Rich Nasdaq Gh Seeking Alpha
 Workflow For The Guardant360 Cell Free Circulating Dna Ngs Genomic Download Scientific Diagram
Workflow For The Guardant360 Cell Free Circulating Dna Ngs Genomic Download Scientific Diagram
 Liquid Biopsy Tailwinds At Last Health Advances Blog
Liquid Biopsy Tailwinds At Last Health Advances Blog
Https Www Cadth Ca Sites Default Files Hs Eh Eh0077 Liquid Biopsy For Early Detection Of Cancer Pdf
 The Biggest Liquid Biopsy Company Files For Ipo Nanalyze
The Biggest Liquid Biopsy Company Files For Ipo Nanalyze
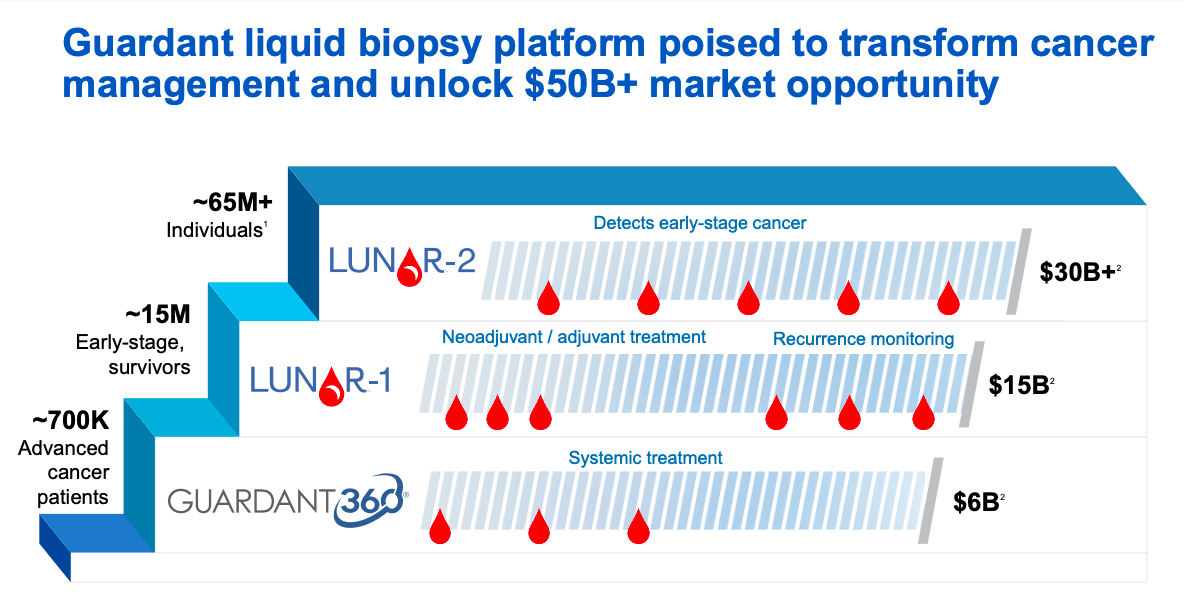 Guardant Health A Rising Star Nasdaq Gh Seeking Alpha
Guardant Health A Rising Star Nasdaq Gh Seeking Alpha
Http Simposiobiopsialiquida Com Wp Content Uploads 2018 01 2 Dr Emiliano Calvo Pdf
 The Biggest Liquid Biopsy Company Files For Ipo Nanalyze
The Biggest Liquid Biopsy Company Files For Ipo Nanalyze
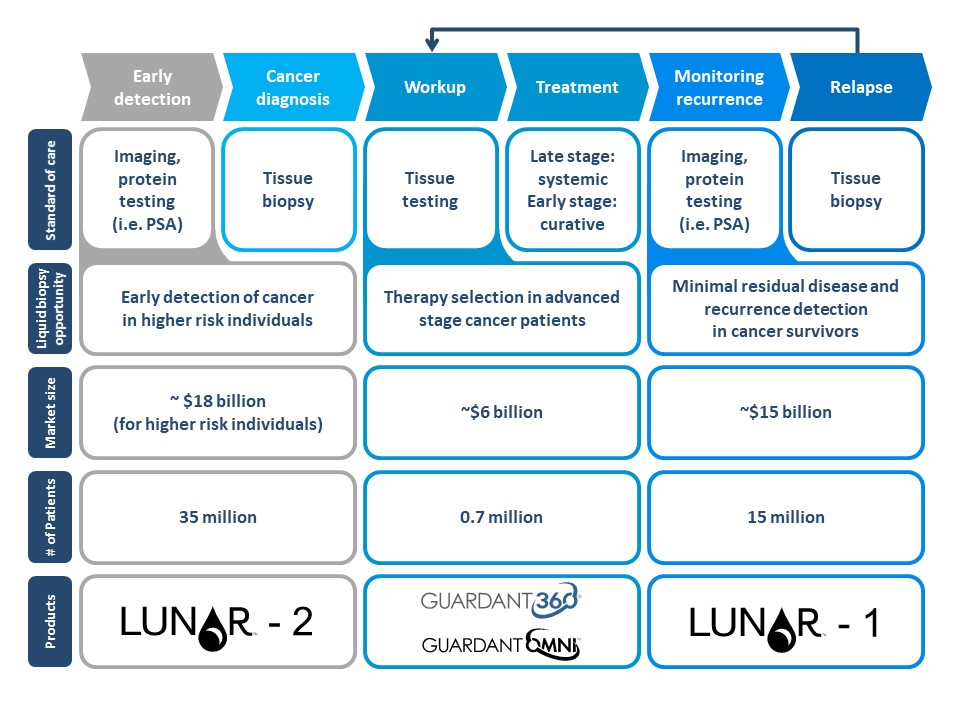
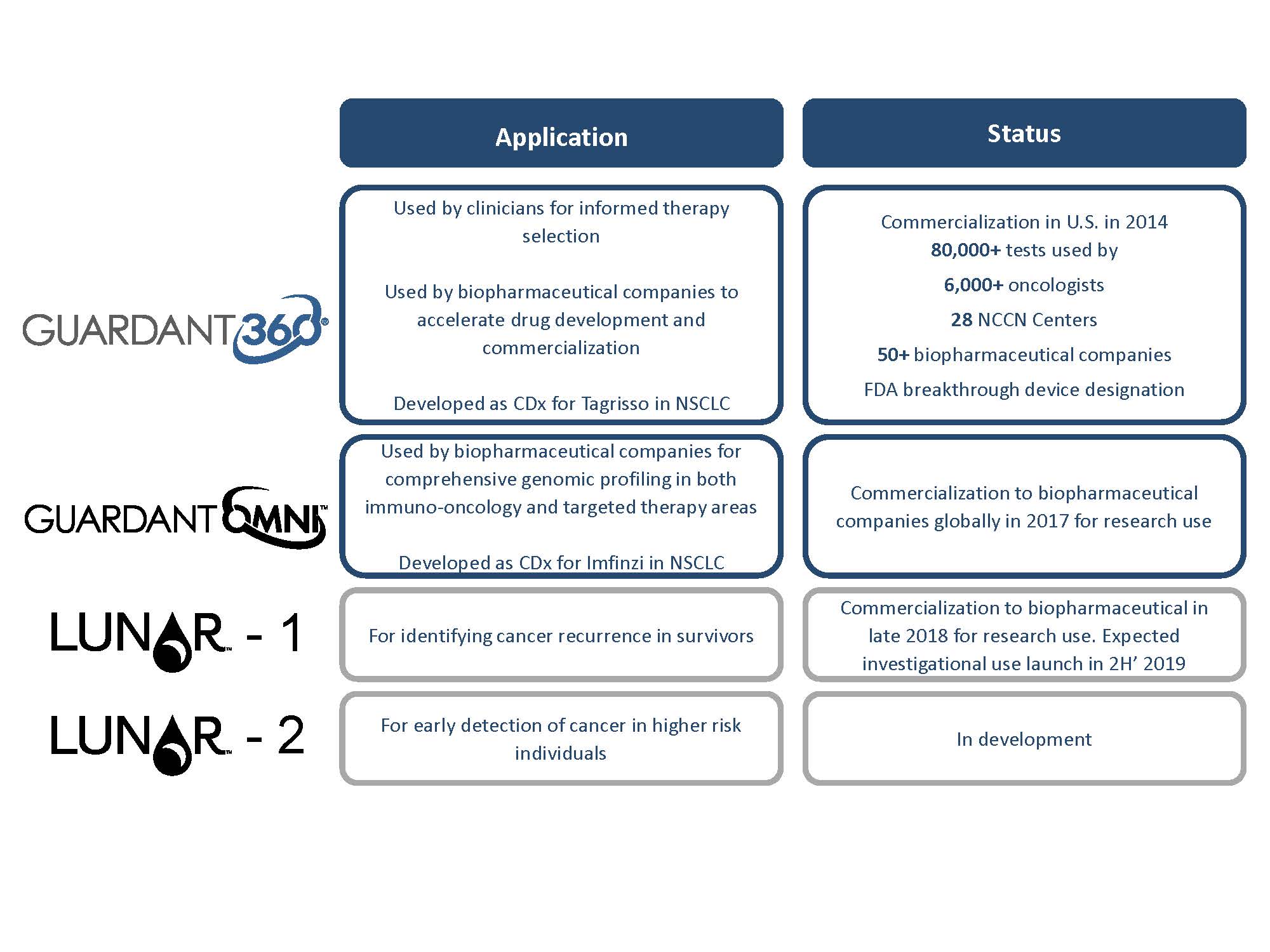

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.